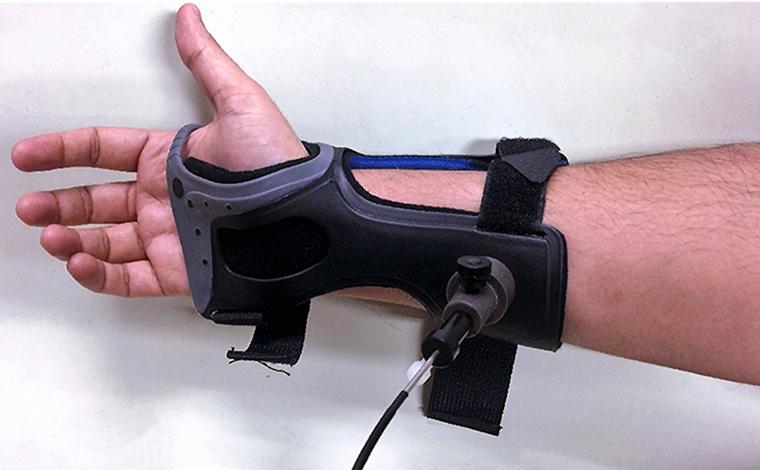
There has never been a way for people with diabetes to test their glucose levels without pricking their body in some form. However, one MU medical professional has recently done work to try to change that.
Anandhi Upendran, director of biomedical innovations at MU School of Medicine Institute for Clinical and Translational Science, recently conducted a study for a non-invasive glucose test device.
Upendran worked in conjunction with Jeon Woong Kang, a research scientist for Massachusetts Institute of Technology Laser Biomedical Research Center and co-author of the study.The two conducted the research at MU where Upendran recruited 20 volunteers through flyers and an MU System-wide email.
“We recruited human volunteers for the study and made sure that the glucose measurement values were best correlated with that of the fingertip measurement that is regularly used,” Upendran said.
The device was developed at MIT and uses a laser scanner connected to the patient’s wrist. The device uses a technique known as Raman spectroscopy, which scans the skin to measure glucose levels.
Upendran discussed the importance in making the device non-invasive, stating that the constant pricking can create pain for any diabetic.
Upendran’s point about pain is something with which freshman Max Smith agreed. Smith, a Type 1 diabetic for nearly 10 years, pricks himself 6-7 times throughout the day in order to make sure his glucose levels are where they need to be. He said this is not something that is easy for him.
“I have scars on the side of finger from doing [pricking] too much,” Smith said.
Smith said having a device that is non-invasive would be an incredible help for the diabetic community, as well as himself.
Smith also expressed interest in other new technology, such as the CGM G6, which requires no finger pricking and no calibration.
The difference between the device Upendran worked on and the new technology is that the CGM G6 requires an abdominal prick.
“The one problem with all devices are that they still need pricking still which can lead to infection,” Upendran said.
Smith is not alone. 1.25 million Americans suffer from Type 1 diabetes with an estimated 40,000 people receiving a diagnosis each year, according to the American Diabetes Association.
Upendran is done working with the device which will go through a clinical trial before seeking FDA approval.
“This study has been performed only in healthy volunteers, in order to make sure that this can go for FDA approval or for a complete or authenticated future we have to do a clinical trial,” Upendran said. “They are looking for patients to take part in it.”
The device has since returned to MIT and allowed Upendran to pick up other projects here at MU.
“I loved working on the project,” Upendran said. “I am glad that it came to a point that we could match or correlate it with a fingerprint in the future. Whatever is done I am confident it will be successful in the diabetic population too.”
_Edited by Morgan Smith | [email protected]_